
CLART STDs is an in vitro diagnostics product used for the detection and typing of bacteria, fungi and parasites causing Sexually Transitted Infections (STIs) of the urogenital tract. Simultaneous detection is performed by multiplex PCR and subsequent visualization in low-density arrays, based on CLART Technology.
Analysis information
CLART STDs detects genetic material extracted from urine or swabs samples. This will get a reduction in overall time of diagnosis, more than 24 hours in many cases, allowing the physician to make adjustments in medication and / or therapies administered to each patient.
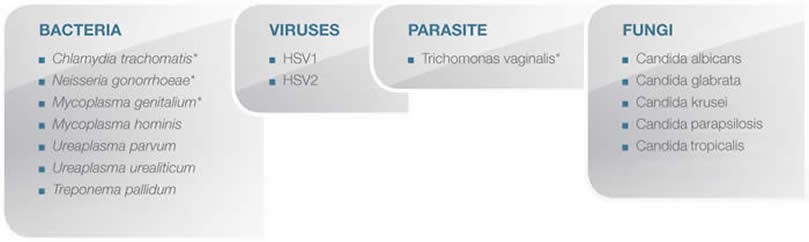
Microorganism detected with CLART STDs
The use of molecular techniques in the detection of sexually transmitted infections reduces the main limitations and disadvantages of conventional detection methods:
Sensitivity variations observed with different diagnostic methodologies.
Low sensitivity to slow-growing microorganisms.
Antibody titer variations due to antiretroviral treatments.
Validated sample types
The kit CLART STDs has been developed and validated for the detection of different causative agents of STDs in the following samples:
Urine.
Swabs: cervical, vaginal, urethral and rectal.
High sensitivity and specificity
The presence of 120 hybridization probes within the array, make it possible to identify, each target at least in triplicate. This ensures specificity and sensitivity of the analysis.
Analysis of results is performed in a fully automatic way by SAICLART, the GENOMICA image processing software for microarrays. This software can detect and interpret all the targets present in the image, thus avoiding any subjectivity that might be introduced by the user. The user thus obtains the results in a fast, simple and reproducible way, presented in clear and concise reports that can be printed or exported to the laboratory information management system.
