Pneumo CLART bacteria is an in vitro diagnostics product for detecting and characterizing multiple bacteria causing repiratory tract infections. Simultaneous detection is performed by multiplex PCR and subsequent visualization in low-density arrays, based on CLART Technology.
Analysis information
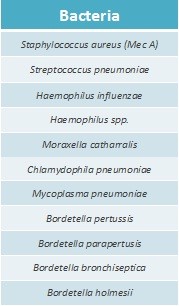
Pneumo CLART bacteria detects the presence of pathogenic and potentially pathogenic bacteria in resp
iratory samples as BAL, BAS, lavages, sputum…
The rapid detection of bacteria allows the clinician to adjust the antimicrobial therapy, thus improving patie
nt’s health and recovery perspectives, as well as reducing associated costs due to prolonged hospitalization or non-effective therapies.
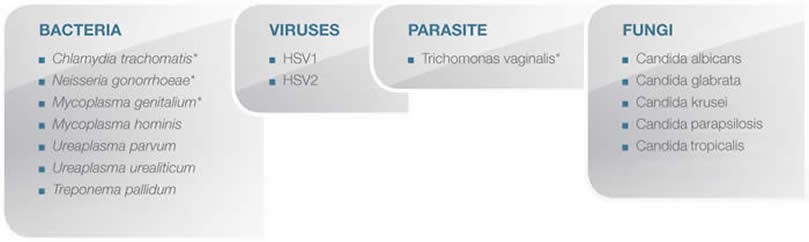
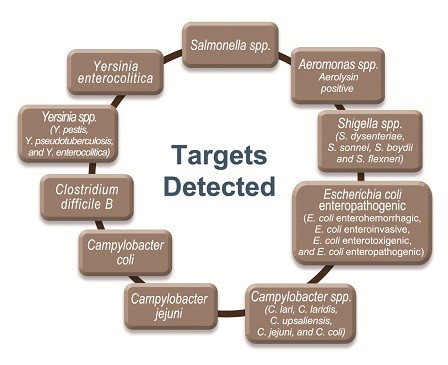
Pneumo CLART bacteria is able to detect the following targets when present in the sample:
Implementation of new molecular technologies in the diagnosis of bacterial respiratory infections considerably reduces the drawbacks of the main traditional detection systems as:
Low sensitivity of some cultures media.
Turnaround time to results.
Highly specific transport media.
Pneumo CLART bacteria has been developed and validated for detection of aforementioned bacterial in the following samples:
Sputum.
Nasopharyngeal lavage, exudate and aspirate.
Broncoalveolar lavage (BAL).
Broncoaspirate (BAS).
Sensitivity and specificity
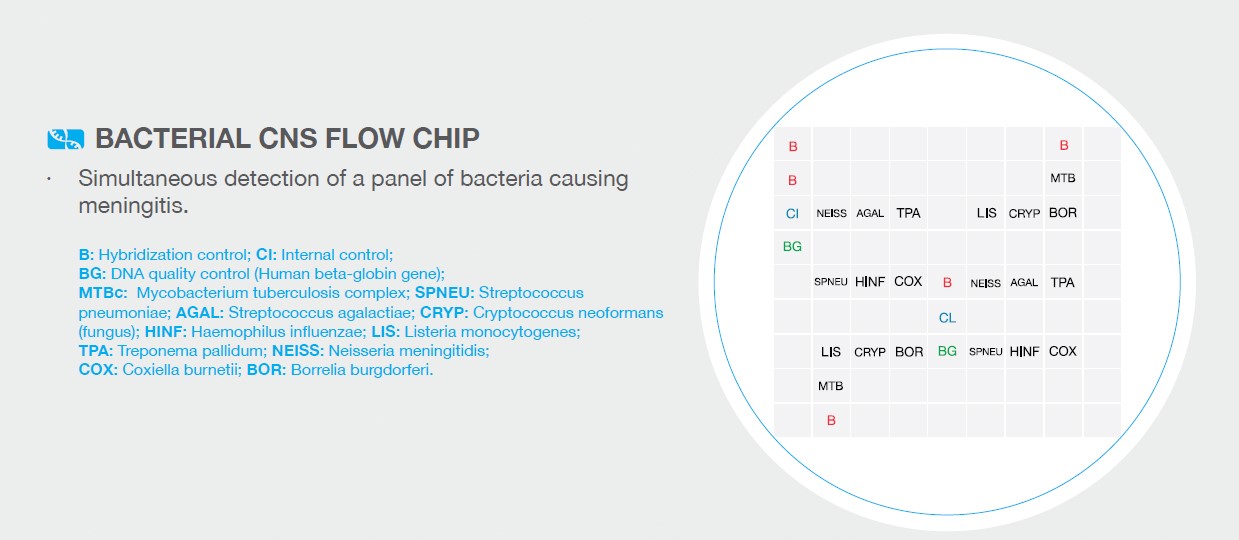
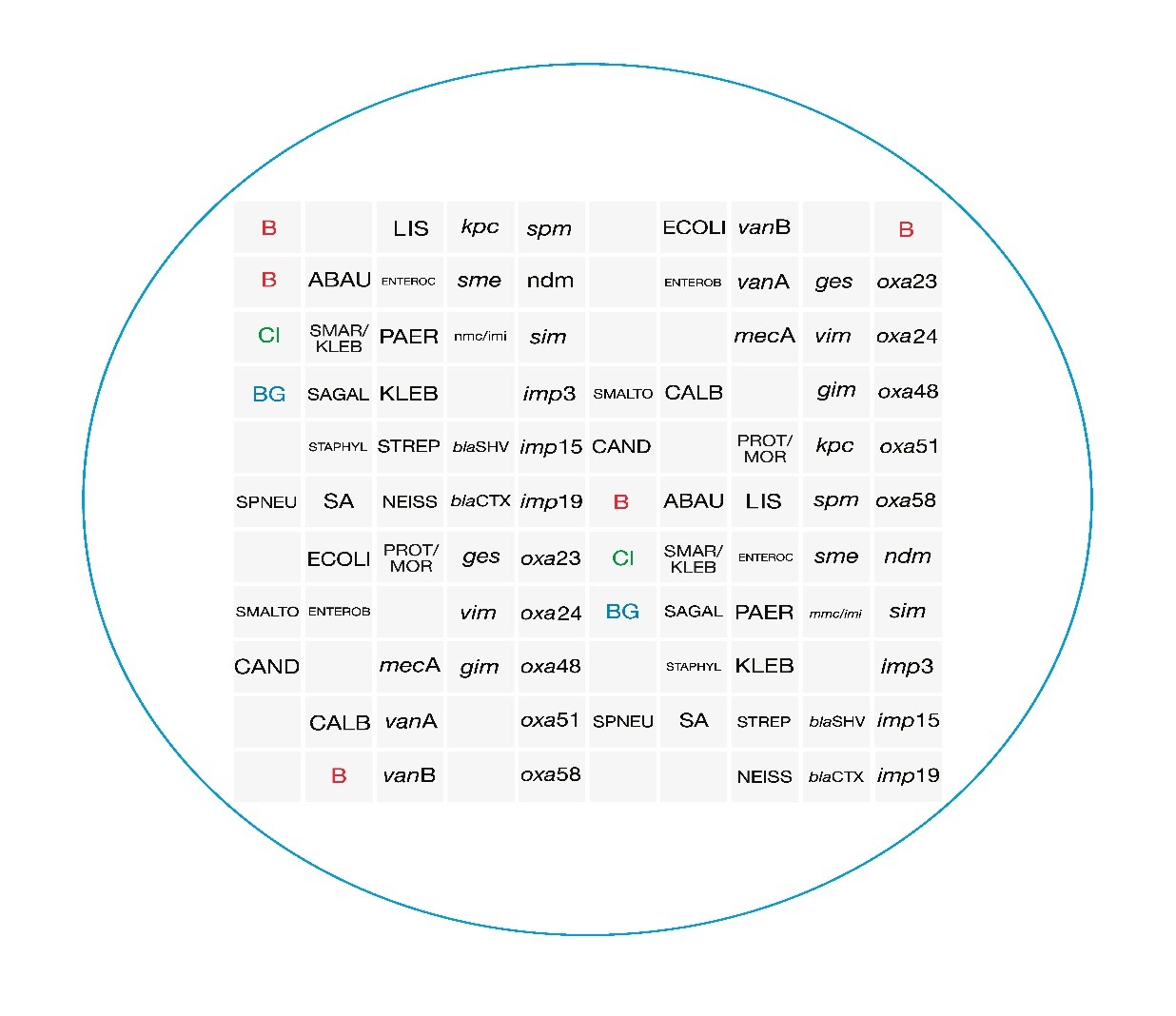
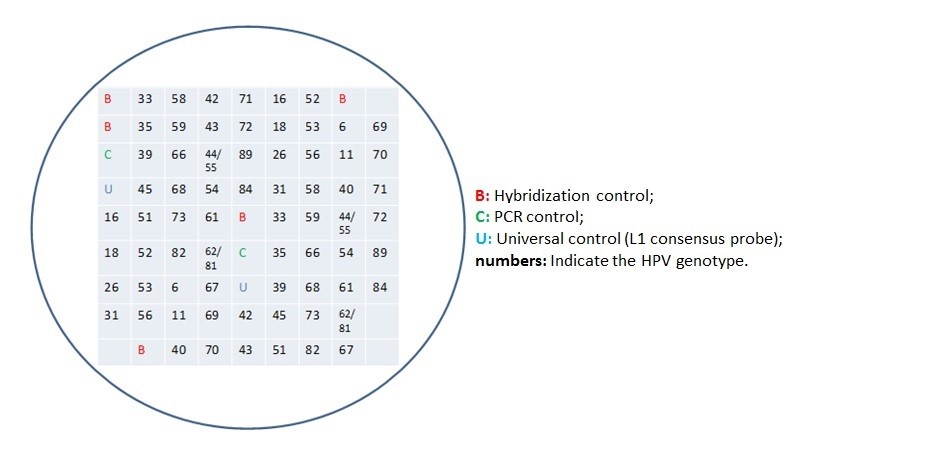
The presence of 120 hybridization probes within the array, allows to identify each target at least in triplicate. This ensure specificity and sensitivity of the analysis.
Quality control
Pneumo CLART bacteria guarantees the quality of the results by including three quality controls in the amplification tubes, thus achieving considerable savings in reagents for parallel checks:
Amplification control on the amplification tube: prevent false negative results.
Endogenous genomic control: certifies the effectiveness of the extraction process.
Biotin markers: they have a dual aim; firstly, act as reference system for the automatic alignment of the array grid and, secondly, serve as control of the reagents performance provided for the visualization step.
Automatic reading and interpretation of results
Analysis of results is performed in a gully automatic way by SAICLART, the GENOMICA image processing software for microarrays. This software can automatically detects and interpret all the targets present in the image, thus avoiding any subjectivity that might be introduced by the user intervention. The user thus obtains the results in a fast, simple and reproducible way, presented in clear and concise reports that can be printed or exported to the laboratory information management system.